Specialized in pharmaceuticals and medical devices
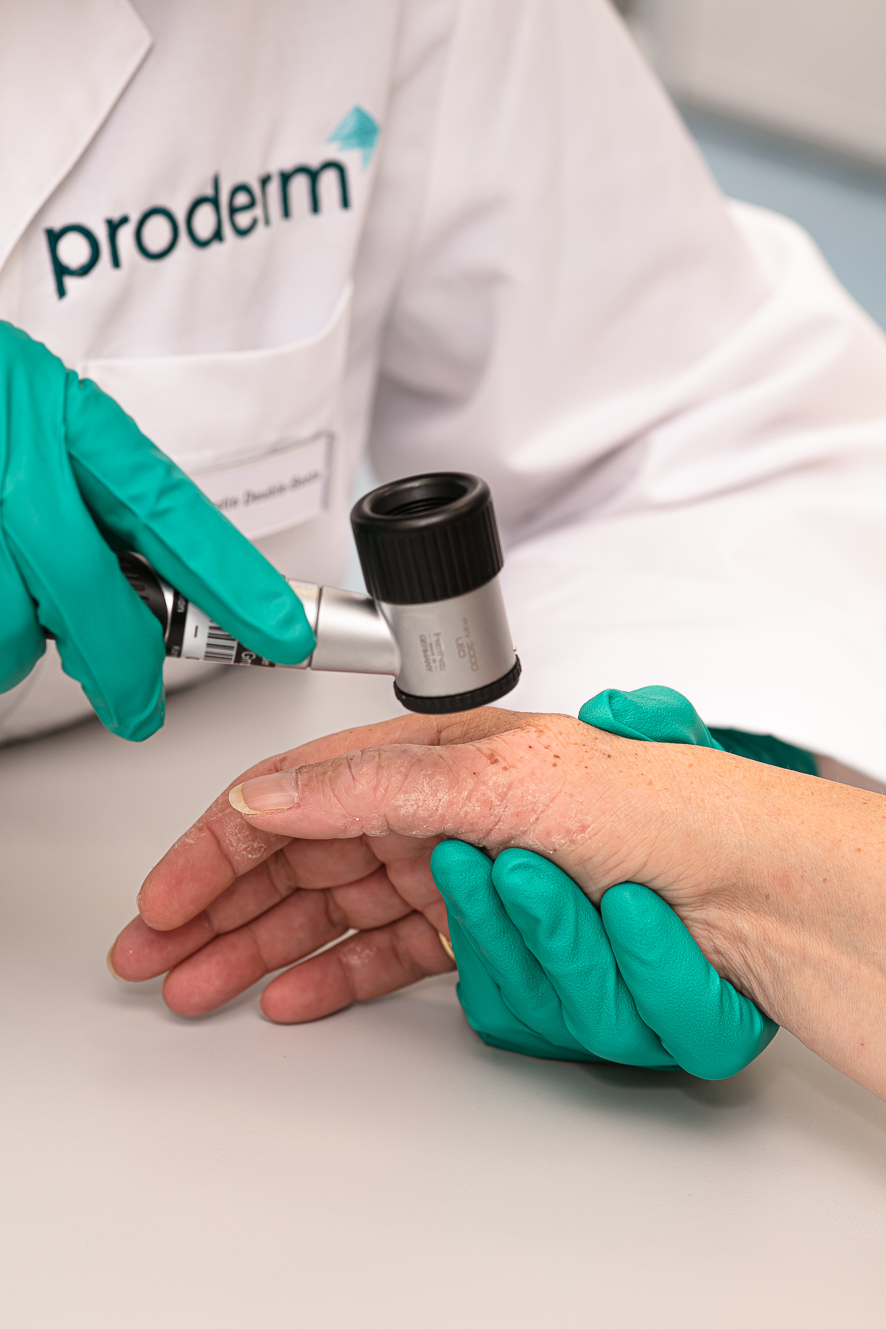
SGS proderm Medical is specialized in clinical trials with drugs and medical devices. As a full-service partner or by taking over individual work packages, we support our clients in all areas, from regulatory consulting, site selection (if required), feasibility analysis, complete study planning (including submission to the relevant authorities) and implementation, data management, statistics, clinical and medical monitoring, through to a sound final clinical report and publication of the data.
Since 1995, we have been conducting clinical trials with medical and scientific excellence according to standard designs and tailored methods in single- and multi-center settings. Our scientific focus is the investigation of systemic and topical preparations for the treatment of diseases or injuries affecting the "surface of the human body" (skin, mucosa, gynecology, oral care, hair, eyes). In addition, SGS proderm also offers clinical studies with other systemic products, such as vaccines.
Your advantages as a customer of SGS proderm Medical
- > 27 years of experience
- Full-service CRO
- SGS proderm-owned study centers with qualified investigators
- Extensive and continuously growing network of pre-qualified study centers
- Patient database with chronic indications (rosacea, psoriasis, atopic dermatitis etc.)
- Highly innovative research and method development
- Collaboration with medical opinion leaders (Medical Experts/KOL)
Customized solutions
We offer custom-fit solutions that are not only flexible but also individually tailored to the customer and continuously invest in the development of new methods and the application of the latest technologies.
SGS proderm Medical works for well-known international customers (biotech, pharma, medical devices) of various sizes and has successfully completed a large number of projects on their behalf, including several thousand patients in various indication areas. We are a member of the Bundesverband Medizinischer Auftragsforschungsinstitute (BVMA) and are regularly audited for compliance with the BVMA's quality criteria.
SGS proderm Medical - Your reliable partner for clinical studies.
Client feedback
- Sponsor feedback following a multicentre alopecia phase IIa trial with more than 200 patients to be recruited: „Fantastic - You have all made a great job making this possible and the number of drop outs are unprecedented low!“
- Sponsor feedback on proderm reaching the randomized 180 patients milestone in a study with a personal lubricant: "This is excellent news!! Great work by the proderm team – recruitment has been completed ahead of schedule."